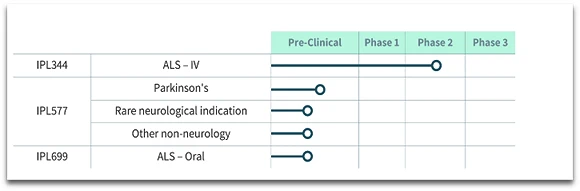
IPL344
 IPL344 is a novel peptide that reactivates the impaired Akt pathway without mediation of the cell membrane receptors that are damaged in ALS. Akt activity has been shown to be down-regulated in post-mortem analysis of ALS patients motor neurons, as well as in neurons and muscles of ALS animal models. By activating the Akt pathway, treatment with IPL344 may address key ALS pathologies including apoptosis, cellular stress, protein misfolding, glucose metabolism and inflammation.
IPL344 is a novel peptide that reactivates the impaired Akt pathway without mediation of the cell membrane receptors that are damaged in ALS. Akt activity has been shown to be down-regulated in post-mortem analysis of ALS patients motor neurons, as well as in neurons and muscles of ALS animal models. By activating the Akt pathway, treatment with IPL344 may address key ALS pathologies including apoptosis, cellular stress, protein misfolding, glucose metabolism and inflammation.
Preclinical studies have demonstrated that IPL344 treatment improved neuro-muscular function in SOD1G93A ALS model mice, reduces weight loss and significantly extended their survival.
IPL344 is being evaluated in an open-label phase 1/2a clinical trial with ALS patients at the Hadassah Medical Center in Israel. The drug is given daily as bolus intravenous administration in a short and simple procedure at patient’s home by family members and caregivers.
So far, IPL344 has demonstrated an excellent safety and tolerability profile in study participants. Efficacy results demonstrate clinically significant slowdown in several disease parameters and clinical endpoints demonstrated by different assessment methods.
Immunity Pharma Announces Positive Top Line Results from Phase 2a Trial with IPL344 in ALS Patients
Moreover, reduction of plasma concentrations of neurofilament-light (NfL) following IPL344 treatment indicated on slowdown of neuronal degeneration in those patients.
![]() ImmunityPharma NfL December 2023 Poster.pdf
ImmunityPharma NfL December 2023 Poster.pdf
IPL344 has an Orphan Drug Designation in ALS, from FDA and EMA.
Preclinical Projects
Additional projects based on IPL344 and other Akt activators are currently in the research/pre-clinical stage.